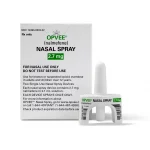
The U.S. Food and Drug Administration on Monday approved a second nasal spray for reversing an opioid overdose. To be sold as Opvee, the spray contains the medication nalmefene hydrochloride and will be available to Americans aged 12 and older with a prescription, the FDA said. “The agency continues to advance the FDA Overdose Prevention Framework and take actionable steps that encourage harm reduction by supporting the development of novel overdose reversal products,” FDA Commissioner Dr. Robert Califf said in an agency news release. “On the heels of the FDA’s recent approval of the first over-the-counter opioid reversal agent [Narcan], the availability of nalmefene nasal spray places a new prescription opioid reversal option in the hands of communities, harm reduction groups and emergency responders,” Califf added. Indivior, which will make and sell Opvee going forward, said the spray should be available by October. Indivior bought Opiant Pharmaceuticals, which developed Opvee, in March. “Opvee’s FDA approval represents a significant achievement in the development of new treatment options to address today’s era of opioid overdoses that are driven by powerful synthetic opioids, such as fentanyl,” Indivior CEO Mark Crossley said in a company news release. “Opvee is an emergency treatment for the fast reversal of respiratory depression triggered by natural or synthetic opioids, including fentanyl, and we are committed to making this novel rescue medication widely available… read on > read on >