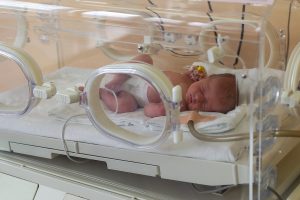
A pregnant woman’s mental health might have profound effects on the mind of her unborn child, a new evidence review warns. Children appear to be at higher risk for mental health and behavior issues if their moms were highly stressed, anxious or depressed during pregnancy, researchers report. In particular, children were more likely to have ADHD symptoms or exhibit aggressive or hostile behavior if their moms reported more anxiety, depression or stress while pregnant. “Our research suggests that psychological distress during the pregnancy period has a small but persistent effect on children’s risk for aggressive, disinhibited and impulsive behaviors,” said researcher Irene Tung, an assistant professor of psychology at California State University, Dominguez Hills. For the review, Tung and her colleagues pooled and analyzed data from 55 studies involving more than 45,000 participants. All the studies measured women’s psychological distress during pregnancy, and then later measured their children’s “externalizing behaviors” — mental health symptoms directed toward others. The researchers also included only research where the mothers’ distress was measured both during and after pregnancy, to control for the potential effects of postpartum mental health problems on developing newborns. They found that even after controlling for mom’s postnatal distress, psychological symptoms during pregnancy independently raise children’s risk of mental health problems. The effect held true for both boys and girls. It was strongest in early childhood… read on > read on >






















-300x200.jpg)