
Agency revokes previous authorization for color additive to be used in food and ingested drugs
read on >

Agency revokes previous authorization for color additive to be used in food and ingested drugs
read on >
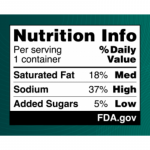
Grabbing a quick snack might soon come with a little extra clarity. The U.S. Food and Drug Administration (FDA) has proposed a new rule requiring bold, easy-to-read nutrition labels on the front of food and beverage packages. These labels, which would highlight content of sugar, salt, and saturated fat, aim to make it easier for shoppers to make healthier choices in the grocery aisle — helping to tackle the rising rates of obesity and conditions such as Type 2 diabetes, heart disease, and high blood pressure, a media report from The New York Times states. These chronic illnesses affect over 60% of American adults and contribute to an estimated $4.5 trillion in annual health care costs, according to the FDA. “Nearly everyone knows or cares for someone with a chronic disease that is due, in part, to the food we eat,” Dr. Robert Califf, the commissioner of the FDA, said in a statement released by the administration. “It is time we make it easier for consumers to glance, grab and go.” The black-and-white labels would appear on the front of products, unlike the current back-of-package Nutrition Facts panel, which lists dietary facts such as calorie counts, serving sizes, and ingredients. The new proposal is the result of three years of research by FDA scientists, who studied similar front-of-package labeling systems used in countries like Canada,… read on > read on >
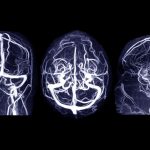
Eating disorders appear to be linked to differences in brain structure among teenagers. Young adults who develop eating disorders appear to have delayed brain maturation as teenagers, MRI scans show in a new study published Jan. 10 in the journal Nature Mental Health. In particular, reduced maturation of the cerebellum — a brain region that controls appetite — helped explain an increased risk of unhealthy dieting or purging by age 23, researchers said. “Our findings reveal how delayed brain maturation during adolescence links genetics, mental health challenges and disordered eating behaviors in young adulthood, emphasizing the critical role of brain development in shaping eating habits,” lead researcher Xinyang Yu, a doctoral student at the King’s College London Institute of Psychiatry, Psychology & Neuroscience, said in a news release from the university. For the study, researchers analyzed data from nearly 1,000 people in England, Ireland, France and Germany. The participants all underwent MRI scans at ages 14 and 23, provided samples for genetic analysis, and completed questionnaires related to their eating habits. By 23, about 42% of the participants had healthy eating behaviors, 33% tended to diet and purge, and 25% were binge eaters, researchers noted. Eating disorders were linked to emotional problems in their teenage years like anxiety and depression, researchers found, as well as behavioral problems like hyperactivity. Anxiety and depression also significantly increased… read on > read on >

The words “calm down” are worse than unhelpful — they actually can increase blood pressure among new mothers of color, a study has found. Gender-based racism through such microaggressions significantly increased a new mom’s blood pressure, compared to women not subjected to these sort of comments, researchers reported in a study published Jan. 9 in the journal Hypertension. And effects on blood pressure were even more pronounced among women living in areas with high levels of structural racism. “It is well-known that Black, Hispanic and South Asian women experience microaggressions during health care. It is not as well known whether these microaggressions may have an association with higher blood pressure,” lead researcher Teresa Janevic, an associate professor of epidemiology at Columbia University Mailman School of Public Health in New York, said in a news release from the college. For the study, researchers asked nearly 400 women of color who gave birth at four hospitals in Philadelphia and New York City about the microaggressions they faced during their care. The women ranted in age from 16 to 46, with about 43% between 20 and 29. Examples included “I have been disrespected,” “Someone told me to calm down,” and “Someone accused me of being angry when speaking assertively.” Nearly two in five women (38%) reported at least one instance of microaggression during their pregnancy care, results show.… read on > read on >

The Mediterranean diet is renown for its ability to improve heart health and help folks lose weight. Now a new rat study says this eating pattern also might provide folks a boost in brain power. Lab rats fed a Mediterranean diet developed changes in gut bacteria that researchers linked to better memory and improved cognitive performance, according to results published recently in the journal Gut Microbes Reports. “Our findings suggest that dietary choices can influence cognitive performance by reshaping the gut microbiome,” lead researcher Rebecca Solch-Ottaiano, a neurology research instructor at Tulane University’s Clinical Neuroscience Research Center, said in a news release from the college. For the study, researchers fed rats a diet rich in olive oil, fish and fiber over 14 weeks. The young rats were approximately equivalent in age to 18-year-old humans. The rats showed increases in four beneficial types of gut bacteria, compared to another group of rats eating a Western-style diet high in saturated fats. These changes in gut bacteria were linked to improved performance on maze challenges designed to test the rats’ memory and learning abilities, researchers said. The Mediterranean diet group also showed better cognitive flexibility, or the ability to adapt to new information, results show. They also had better short-term “working” memory. These results suggest that teenagers and young adults whose brains and bodies are still maturing could… read on > read on >
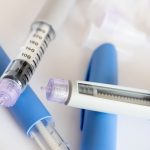
Three GLP-1 drugs are best at helping obese and overweight people drop weight, including one that hasn’t yet been approved for that purpose. A new evidence review published in the Annals of Internal Medicine shows that the widely-known drugs tirzepatide (Zepbound) and semaglutide (Wegovy) are both safe and effective at helping the obese shed pounds. However, a third drug also FDA-approved for weight loss, liraglutide (Saxenda), didn’t work as well as the others, researchers found after reviewing data from dozens of clinical trials. The review also found that a GLP-1 drug still in development, called retatrutide, might be more effective than either Zepbound or Wegovy. “We found that, of the 12 GLP-1 (drugs) identified by our search, the greatest mean body weight reduction was reported in randomized controlled trials of retatrutide, tirzepatide, and semaglutide,” concluded the research team led by Dr. Mark Eisenberg, a professor of medicine at McGill University in Montreal, Canada. GLP-1 drugs, initially developed to treat diabetes, help people lose weight by slowing digestion in the stomach and sending signals to the brain indicating that a person has eaten enough and feels full. The drugs mimic a hormone secreted by the small intestine when people eat food. For the new evidence review, researchers analyzed data from 26 previous clinical trials of the drugs, involving nearly 15,500 participants. Compared to placebo, the data… read on > read on >

The U.S. Food and Drug Association (FDA) released the first-ever guidelines for levels of lead in processed baby foods this week. However, many health and safety advocates say they are not satisfied with the guidance. Under the FDA’s new guidelines, baby food manufacturers should have no more than 10 parts per billion of lead in baby yogurts, custards, puddings, single-ingredient meats, processed fruits and vegetables, and mixtures of fruits, vegetables, grains and meat. Yet the new guidance does not cover many other products, such as infant formula, beverages, or snack foods like puffs and teething biscuits. “Nearly all baby foods on the market already comply with these limits,” Jane Houlihan, research director of Healthy Babies Bright Futures (HBBF), told CNN. HBBF is a coalition of advocates committed to reducing babies’ exposures to neurotoxic chemicals. Houlihan said the newly released FDA guidelines were ineffective — not to mention unenforceable. In 2019, HBBF released a report that found toxic metals in 95% of baby foods randomly pulled off supermarket shelves. It led to a congressional investigation that discovered some baby food ingredients contain hundreds of parts per billion of dangerous metals, according to internal documents provided by major baby food manufacturers. “As it stands, the new lead limits for commercial baby foods would reduce children’s total dietary lead exposure by less than 4% — a negligible improvement,”… read on > read on >

A new diet is on the New Year’s resolution list for nearly half of U.S. adults, according to a new survey from the Physicians Committee for Responsible Medicine. However, many plan to pick up diets with dicey track records, survey results show. About 46% of adults said they plan to start a new diet in 2025, the survey found. When asked which diets they plan to try, results show that: 40% said they’ll eat fewer calories. 26% said they’ll try a low-carb diet like keto, Atkins or South Beach. 7% said they’ll eat a plant-based diet. Research has shown that a plant-based or vegan diet is among the most effective means for losing weight and keeping it off, experts said. “Counting calories can be time consuming and create a negative relationship with food for some people, and low-carbohydrate diets come with a range of side effects,” Dr. Roxanne Becker, medical editor with the Physicians Committee, said in a news release from the group. “Research has shown that plant-based diets are effective for weight loss without purposefully restricting or counting calories,” Becker continued. “This is because plants tend to be naturally lower in calorie density and higher in fiber, which promotes a feeling of fullness.” The committee pointed to a 2017 study in which obese or overweight New Zealand residents ate a whole-food, plant-based diet for… read on > read on >

Weight loss tops many folks’ list of New Years resolutions, and lots of people are turning to cutting-edge weight-loss drugs like Ozempic to help them drop excess pounds. These drugs, called glucagon-like peptide-1 receptor agonists (GLP-1 RAs), work in several different ways to help people lose weight, gain control over their blood sugar levels, and improve their heart health, a new study published in the journal Cureus says. GLP-1 drugs mimic a hormone secreted by the small intestine when people eat food. The drugs initially were approved as a treatment for type 2 diabetes because they help lower blood sugar levels. But subsequent studies found that GLP-1 drugs also help people lose weight. The new study noted the different means by which GLP-1 drugs act upon the body. Specifically, these drugs: Increase insulin production, which lowers blood sugar Delay digestion in the stomach, reducing hunger levels Send signals to the brain indicating that a person has eaten enough and feels full Promote the breakdown of cholesterol in the bloodstream, reducing a person’s heart risk Lower blood pressure by inhibiting inflammation in the blood vessels Reduce blood sugar production in the liver, protecting the organ from potential scarring “All these emerging benefits have made GLP-1 RAs an important pharmacological drug,” concluded the research team led by senior researcher Dr. Zahra Nazir with the Combined Military Hospital… read on > read on >

The U.S. Food and Drug Administration (FDA) has proposed a new rule to require standardized testing of talc-containing cosmetics for asbestos, a known carcinogen linked to serious illnesses such as lung and ovarian cancers. According to an FDA report, the proposed rule would mandate that manufacturers test each batch of talc-containing cosmetic products using advanced microscopy techniques, including polarized light and transmission electron microscopy. Failure to comply with these testing or record-keeping requirements would result in the products being classified as adulterated under the Federal Food, Drug, and Cosmetic Act. Asbestos contamination in talc-based cosmetics has been a long-standing health concern. The FDA emphasized there is no safe level of asbestos exposure, and this rule aims to reduce harmful exposure and protect consumers from potential health risks. The FDA’s announcement comes as Johnson & Johnson faces lawsuits from over 62,000 claimants alleging asbestos in its talc products caused cancer. The company has denied the allegations, calling its products safe, and is working to resolve claims through a $10 billion settlement in bankruptcy. The proposed rule is now open for public and industry comments for 90 days before being finalized, according to a news release from CNN. More information The Occupational Safety and Health Administration has more on asbestos. SOURCE: U.S. Food and Drug Administration (FDA), news release, Dec. 26, 2024; CNN read on >