The herbal supplement kratom regularly causes serious side effects and doesn’t appear safe for use, a new study argues.
Kratom, made from the leaves of a Southeast Asian plant, is usually used to treat pain and addiction. But poison control center data shows it has been tied to seizures, withdrawal, hallucinations, agitation and rapid heart rate, researchers report.
Kratom is “probably not something that’s safe enough to be available as an herbal supplement,” concluded lead researcher William Eggleston, a clinical assistant professor with the Binghamton University School of Pharmacy in New York.
Kratom contains compounds that act on the opioid receptors in the brain and the body, according to the U.S. National Institute on Drug Abuse.
And while it’s a legal herbal supplement, the U.S. Food and Drug Administration has already issued a warning against using kratom. The FDA has called the drug “opioid-like” and cited concerns that it might pose an addiction risk.
In the new study, poison control centers received more than 2,300 calls related to kratom between 2011 and 2018.
Those calls increased from 18 in 2011 to 357 in the first seven months of 2018, according to stats drawn from the U.S. National Poison Data System.
The research team zeroed in on 935 cases where kratom was the only substance involved.
About 56% of cases involved kratom taken as a pill, capsule or powder, and in nearly 9 in 10 cases people ate the kratom that had affected them.
The most commonly reported adverse effects were agitation (in almost 20% of cases), rapid heart rate (17%), drowsiness (14%) and vomiting (11%), the data showed.
Severe side effects included seizures (6%), hallucinations (5%), respiratory depression (3%), and coma (2%). Cardiac or respiratory arrest was reported in 0.6% of cases.
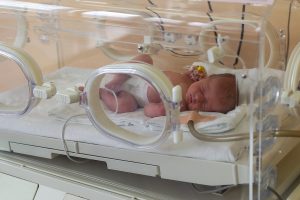
The researchers also identified four cases of neonatal abstinence syndrome, where babies were born addicted to kratom after their mothers took the supplement during pregnancy. They said it caused two deaths.
The National Poison Data System statistics indicated that kratom has a lower risk for fatal overdose than opioids like heroin or fentanyl, Eggleston said.
“The risk for things like serious respiratory depression is probably less with kratom than it is with other opioids,” Eggleston said. “We saw a very low incidence of this in our data.”
However, other studies also have shown that users can experience withdrawal symptoms, Eggleston said.
“That suggests that patients could develop a dependence or a substance use disorder, as you would with other opioids,” Eggleston said. “To me, that exceeds what I would consider a reasonable risk for an herbal supplement you can buy at a local convenience store or head shop.”
Kratom proponents argue that the new study is flawed because it relies on poison control and medical examiner data, which tags kratom as the main suspect and could fail to consider other possible explanations.
“If a person dies and the tox screen identifies kratom in the bloodstream, that is labeled as a kratom-associated death,” said Mac Haddow, a senior fellow on public policy at the American Kratom Association. “It is just as plausible you could identify caffeine in the bloodstream as a result of drinking a cup of coffee that morning.”
Susruta Majumdar, an associate professor with the St. Louis College of Pharmacy in Missouri, said the new study adds a bit more evidence regarding kratom’s safety, but agreed that its reliance on poison control center data makes for a flawed approach.
Based on available data, Majumdar said, kratom probably is safer that prescription and illicit opioids, but “I think we are getting to a point where we can say it’s addictive.”
Majumdar added that he believes kratom-related deaths are not caused by kratom alone, but kratom combined with other substances.
“People are on multiple drugs, and it’s the synergy between those drugs that is causing the toxicity,” Majumdar said.
Eggleston said he does not advocate a ban on kratom, since studies suggest it might have a role in treating chronic pain and addiction.
Instead, clinical trials are needed to assess kratom’s usefulness and establish its safety at certain doses, Eggleston said.
“Our research is not coming from a place where we want to hinder access,” Eggleston said. “We want the public to have all the information they need and be transparent, so they know what works and what’s safe.”
The study findings were published July 9 in the journal Pharmacotherapy.
More information
The U.S. National Institute on Drug Abuse has more about kratom.
Copyright © 2025 HealthDay. All rights reserved.