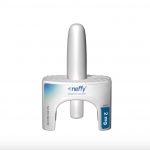
In a surprising move, the U.S. Food and Drug Administration (FDA) has opted not to approve a needle-free alternative to the EpiPen for emergency treatment of severe allergic reactions. Approval of the Neffy nasal spray was widely anticipated. An FDA advisory panel voted to recommend approval of the drug for children and adults in May. While the FDA is not obligated to follow the advice of their advisory panels, it usually does. Instead, the FDA told the drug’s maker, ARS Pharmaceuticals, that it needed to conduct another study on the drug before it is approved, the company said in a statement late Tuesday night. “We are deeply disappointed that this action further delays the availability of Neffy for the millions of people who are at risk of a potentially life-threatening severe allergic reaction,” said Richard Lowenthal, co-founder, president and CEO of ARS Pharma. “We stand by the totality of the Neffy data package in a comprehensive registration program that was aligned upon with FDA and believe strongly in the value Neffy can provide for patients, families and caregivers living daily with severe allergic reactions,” he said in a company statement, adding that his firm will aim to complete the requested trial as soon as possible. The news was unwelcome on the front lines of health care. “It’s certainly disappointing as we were hoping to have… read on > read on >